|
In a lead
acid industrial battery, the conversion of the chemical energy of a battery into electrical energy through “Redox” (oxidation/reduction
reaction), and the withdrawal of the electrical energy into a load.
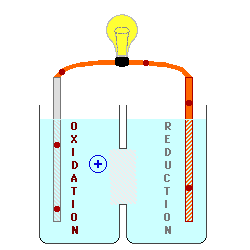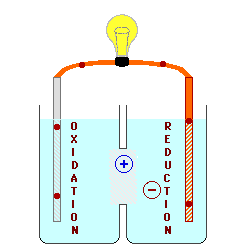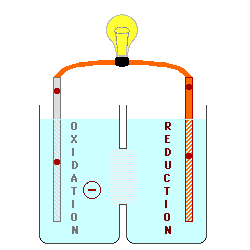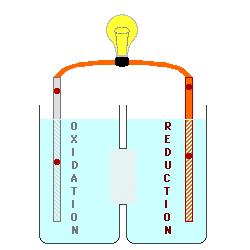
Oxidation describes the loss
of electrons by a molecule, atom or ion.
Reduction describes the gain
of electrons by a molecule, atom or ion.
RELATED ITEMS:
Battery – Defined: http://giantbatteryco.com/GLOSSARY/Battery-Industrial.Batteries.html
Charge: http://giantbatteryco.com/GLOSSARY/Charge-Industrial.Batteries.html
Cycle: http://giantbatteryco.com/GLOSSARY/Cycle.Material-Industrial.Batteries.html
Electric Charge: http://giantbatteryco.com/GLOSSARY/Electric.Charge-Industrial.Batteries.html
Electrochemistry: http://giantbatteryco.com/GLOSSARY/Battery.Electrochemistry-Industrial.Batteries.html
Lead Acid Batteries: http://giantbatteryco.com/GLOSSARY/How.Batteries.Work-Industrial.Batteries.html
Oxidation: http://giantbatteryco.com/GLOSSARY/Oxidation-Industrial.Batteries.html
 Back to top Back to top
|