|
A battery consists of one or
more electrochemical
cells. Each cell contains two metal electrodes and at least one electrolyte
solution (a solution containing ions that can conduct electricity). The
battery operates through electrochemical
reactions called oxidation and reduction. These reactions involve the
exchange of electrons between chemical species. If a chemical species loses
one or more electrons, this is called oxidation.
The opposite process, the gain of electrons, is called reduction.
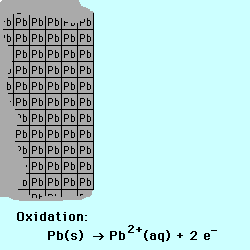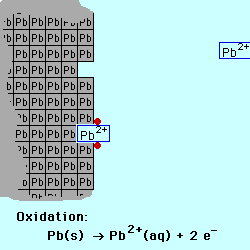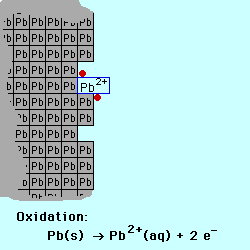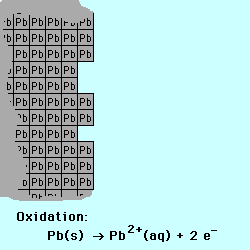
Oxidation occurs at the Anode.
Reduction occurs at the Cathode.
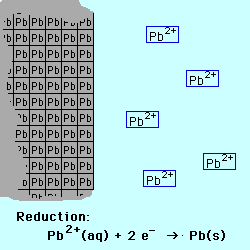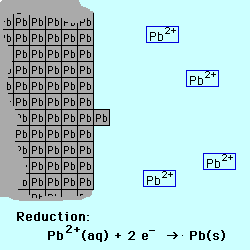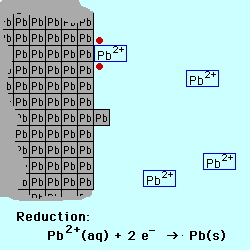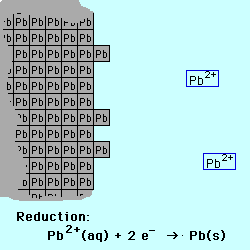
If the reactive
components of an electrochemical cell
are placed in contact with each other, they will react by direct transfer
of electrons (an
oxidation - reduction reaction) and there is no way to harness this energy to do electrical
work. Most of the energy of the reaction is released as heat.
The heat released is closely related to the standard enthalpy change (delta-H°) of the reaction.
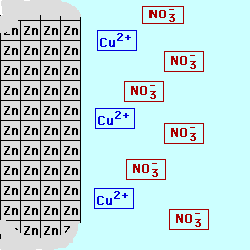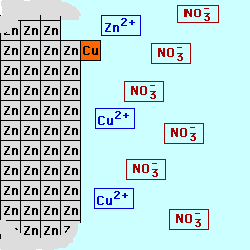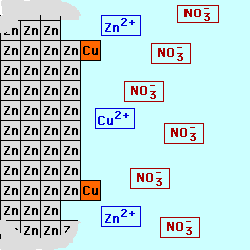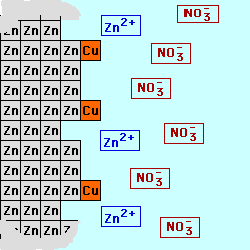

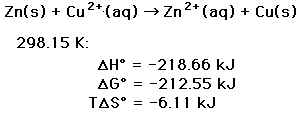
In most batteries, there are
different materials at the two electrodes, such that they want to react
with one material being oxidized and the other being reduced. In the
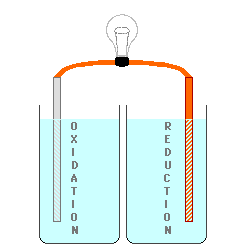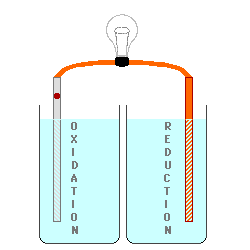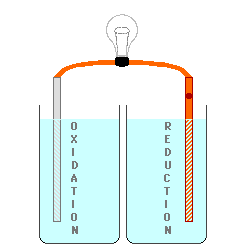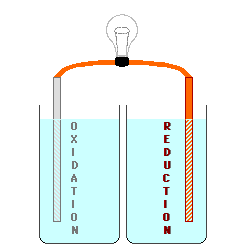
cell below, Zinc is used for the
electrode on the left (the Anode) in contact with a solution of Zinc (II) ions, possibly a solution of Zinc Nitrate. Copper
is used for the electrode on the right (the Cathode) in contact with a
solution containing Copper (II) ions,
perhaps Cupric Nitrate. By keeping
the materials separated, the electrons being produced by the oxidation at
the Anode could be used to do electrical work as they are transferred to
the Cathode where they will be consumed by the reduction process. The
amount of electrical work that a battery may produce is closely related to
the standard free energy change (delta-G°)
of the reaction.
However, the oxidation process
either produces positive ions or removes negative ions from the solution at
the anode (or it may change one ion to a more positive one), and the
reduction process either removes positive ions or produces negative ions in
the solution at the cathode. This produces electrically charged
solutions, and very quickly stops the process before a measurable number of
electrons are transferred.
There must be a path for the
ions to move between the two solutions in order for electrons to flow
continuously through the wire. This produces an "ion current" within the battery with cations (positively - charged ions) moving
from anode to cathode, and anions
(negatively - charged ions) moving from the cathode toward the anode.
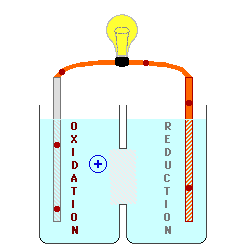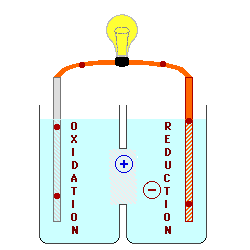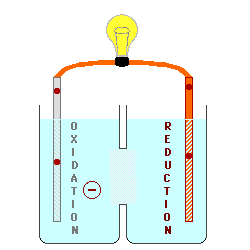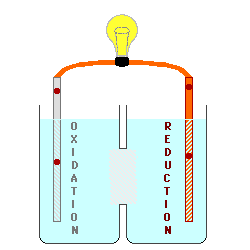
This path may be provided by
having the two solutions in contact with each other, but this allows
diffusion of all of the ions and "runs down" the battery pretty
quickly. This diffusion can be slowed down by separating the
solutions with a membrane or a porous plug. All of these can lead to
a "liquid junction potential"
due to differing rates of movement by the cations and anions. A
"salt bridge" can be used to separate the two solutions with a
third concentrated solution of well - matched cations and anions,
completely eliminating the "liquid junction
potential". In a few cases, it is possible to design a
battery so that both electrodes can be placed in the same container with
only one solution.
In the cell above, the electrons
are produced by the lead metal being oxidized to lead (II) ions, and by
copper (II) ions being reduced to copper metal. Even with the ions
moving across the boundary between the solutions, there is an increase in
the concentration of lead ions on the left and a decrease in copper ions on
the right. This causes the voltage of the battery to decrease, and
eventually the voltage will decrease to zero. Some batteries are
designed to be re-chargeable by forcing electrons to flow backwards through
the cell, reversing the chemical reaction.
The
voltage of a cell may depend on many factors: the electrode materials, the
components and concentrations of the solutions, the type of liquid
junction, the temperature, and the pressure. The voltage also depends
on the electrical current being drawn from the cell. The voltage (E) and the current (I)
are related to the resistance (R)
through Ohm's Law:
E =
IR
The current
is directly related to the rate at which electrons are pumped through the
wire and any resistances in the circuit. As the resistance is lowered
to zero (a short-circuit), the current increases and the voltage of the
cell decreases to zero. As the resistance is
increased, the current decreases, and the voltage increases toward a
limiting value. In Chemistry, we are primarily interested in
this limiting value, the maximum voltage that the electrochemical cell can
deliver. This maximum voltage or electrochemical potential is a measure of the maximum electrical work that can be obtained
from the chemical reaction occurring within the cell, and this can be
related to the Gibbs' Free Energy Change
associated with the chemical reaction.
Before we
leave this discussion to discuss the thermodynamics of batteries, we need
to address the effects of concentration on the voltage of a cell. This
can get somewhat complicated and confusing. We are going to avoid
these problems by focusing on cells with a very specific type of chemical
reaction.
*************************************************
The Nernst
Equation describes the effects of concentrations on the maximum
voltage that the chemical reaction can produce by relating the voltage to
the Standard Electrochemical Potential (E°).
This Standard Electrochemical Potential
represents the maximum voltage the reaction can produce with all of the
components in their standard states or
at unit activity.
*************************************************
The
remainder of this discussion will be concerned with electrochemical
cells that do not involve changes in the concentrations of ions or gases.
In these cells, the Standard Electrochemical
Potential can be measured directly.
One way to do this is by using Metal/Metal Salt electrodes which are prepared
by coating the metal with one of its insoluble salts (or an oxide), as in Silver/Silver Chloride, Lead/Lead Sulfate, or Mercury/Mercurous
Chloride (Calomel) electrodes. These
are usually a solid metal and a solid salt, though in the case of Mercury, the metal
is a pure liquid. Electrical contact is usually made through a platinum
wire in contact with the mercury.
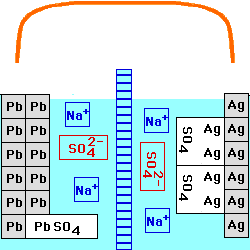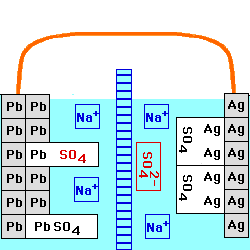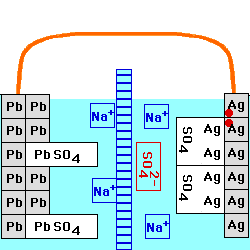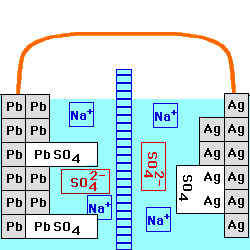 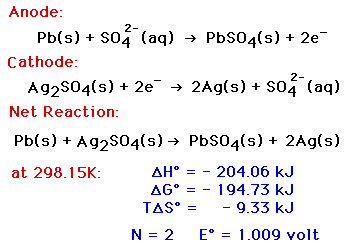
This cell is constructed with a Lead/Lead Sulfate anode and a Silver/Silver Sulfate cathode, both in a
solution of Sodium Sulfate. The
two solutions are separated by an anion
exchange membrane, which allows negatively - charged ions to go
through it, but positively - charged ions cannot. The voltage of this
cell still depends on the current being drawn from it, and on the
temperature. At any fixed temperature, however, the maximum voltage
(at very low current) is independent of the concentration of the
electrolyte, and is equal to the Standard
Electrochemical Potential for this reaction.
Redox (shorthand for oxidation/reduction
reaction) describes all chemical reactions in which atoms have their
oxidation number (oxidation state) changed.
This can be a simple redox process,
such as the oxidation of carbon to yield carbon dioxide, it could be the
reduction of carbon by hydrogen to yield methane (CH4), or a
complex process such as the oxidation of sugar in the human body, through a
series of very complex electron transfer processes.
The term redox comes
from the two concepts of reduction and oxidation. It can be
explained in simple terms:
- Oxidation describes the loss
of electrons by a molecule, atom or ion
- Reduction describes the gain
of electrons by a molecule, atom or ion
However, these descriptions (though sufficient for many purposes) are
not truly correct. Oxidation and reduction properly refer to a change in
oxidation number — the actual transfer of electrons may never occur. Thus, oxidation
is better defined as an increase in oxidation number, and reduction
as a decrease in oxidation number. In practice, the transfer of
electrons will always cause a change in oxidation number, but there are
many reactions which are classed as "redox" even though no
electron transfer occurs (such as those involving covalent bonds).
 Back to top Back to top
|