|

|

|
Cell
A battery is an electric device
that converts chemical energy into electrical energy, consisting of a group
of electric cells that are connected to act as a source of direct current.
Batteries are made of connected cells encased in a container and fitted
with terminals to provide a source of direct electric current at a given
voltage. A battery is characterised by its chemical composition
(combination of metal(s) and electrolyte used), voltage, size, terminal
arrangements, capacity and rate of capability or more cells. In many
contexts it is common to call a single cell used on its own a “battery”.
In order for a cell or battery to be able to deliver
electrical current to an external circuit, a “potential difference” must
exist between the positive and negative electrodes. The potential
difference (usually measured in volts) is commonly referred to as the
voltage of the cell or battery. A single lead-acid cell can develop a
maximum potential difference of about 2 V under load. A completely
discharged lead-acid cell has a potential difference of about 1.75 V,
depending on the rate of discharge.
The simplest method for the construction of lead-acid
battery electrodes is the flat plate. It is merely a flat plate composed of
pure lead. Since the capacity of a lead-acid battery is proportional to the
surface area of the electrodes that is exposed to the electrolyte, various
schemes are employed to increase the surface area of the electrodes per
unit volume or weight. Flat plates are grooved or perforated to increase
their surface area. A typical flat plate is shown below:
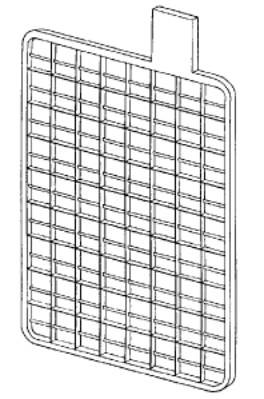
Plate
The most commonly used method to increase surface area is to make
the active material into a paste that acts like a sponge where the
electrolyte fills all the pores. The paste, or active material, is mounted
into a frame or grid structure that mechanically supports it and serves as
the electrical conductor carrying the current during both the charge and
discharge cycle. The most commonly used plate today is the pasted plate,
also known as the flat plate. This grid structure is a latticework that resembles
the cross section of a honeycomb, with the paste filling all of the
rectangular windows on the structure. The picture below shows a typical
construction of a pasted plate grid. The flat plate construction is used as
the negative electrode plate in almost all cases, and serves as the
positive plate in most standby applications.
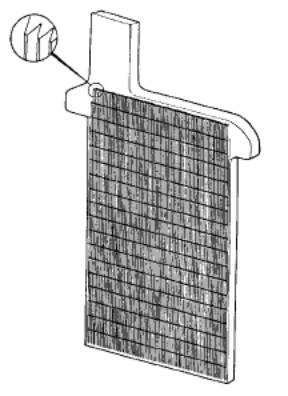
Pasted Grid plate
Positive electrodes are usually of pasted plate or tubular
construction. Tubular electrodes are popular positive plates for heavy cycling
applications. This construction uses a frame structure consisting of a
series of vertical spines connected to a common bus. The paste is held in
micro-porous, non-conductive tubes, which are placed over the individual
spines. A simplified view of tubular plate construction is shown in below.
Regardless of the plate type used, the capacity of any battery is increased
by adding multiple plates in parallel.
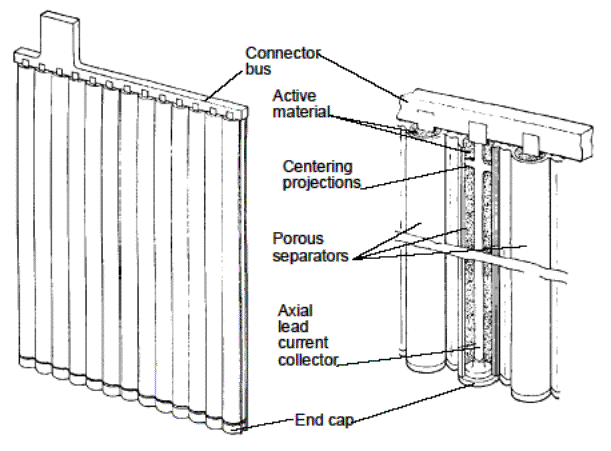
Tubular Plate
 Back to top Back to top
|
Have questions? Contact TECH SUPPORT:
Corporate
|
About Us | Site Map | Services | Clients | Global Presence
| Email: Parts &
Service | Battery Care
Privacy |
Terms of Use |
Ethics |
Legal

Powered by: Sun Microsystems
© Copyright GB Industrial Battery - All rights reserved.
Last Updated: Monday, December 03, 2007 - 6:00 AM Eastern Time.
 
|