|

|

|
Sulfuric
Acid (H2SO4)
|
Sulfuric acid
|
|
|
|
General
|
|
Systematic name
|
Sulfuric Acid
|
|
Other names
|
Battery acid
Electrolyte
Oil of vitriol
|
|
Molecular formula
|
H2SO4
(aq)
|
|
Molar mass
|
98.08 g mol−1
|
|
Appearance
|
Clear, colorless,
odorless liquid
|
|
CAS number
|
[7664-93-9]
|
|
Properties
|
|
Density and phase
|
1.84 g cm−3,
liquid
|
|
Solubility in water
|
Fully miscible
(exothermic)
|
|
Melting point
|
10 °C (283 K)
|
|
Boiling point
|
338 °C (611 K)
|
|
pKa1
|
–3
|
|
pKa2
|
1.99
|
|
Viscosity
|
26.7 cP at 20°C
|
|
Hazards
|
|
MSDS
|
CIS
(ICSC) Card
|
|
EU classification
|
Corrosive (C)
|
|
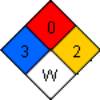
NFPA 704
|
|
|
R (risk)-phrases
|
R35 (causes severe burns)
|
|
S-phrases
|
S1/2 , S26, S30,
S45
|
|
Flash point
|
Non-flammable
|
|
RTECS number
|
WS5600000
|
|
Supplementary data
|
|
Structure &
properties
|
n, εr, etc.
|
|
Thermodynamic data
|
Phase behavior
Solid, liquid, gas
|
|
Spectral data
|
UV, IR, NMR, MS
|
|
Related compounds
|
|
Related strong acids
|
Selenic acid
Hydrochloric acid
Nitric acid
|
|
Related compounds
|
Hydrogen sulfide
Sulfurous acid
Peroxymonosulfuric
acid
Sulfur trioxide
Oleum
|
|
GB Disclaimer:
Except where noted otherwise, data is given for materials in their standard
state (at 25 °C, 100 kPa)
|
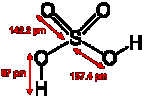
Sulfuric acid, H2SO4, is a strong mineral acid. It is soluble in water at all concentrations.
It was once known as oil of vitriol, and has an oily consistency when
concentrated.
Physical properties:
Forms of
Sulfuric acid
Although nearly 100% sulfuric acid can be made, this loses SO3
at the boiling point to produce 98.3% acid. The 98% grade is more stable in
storage, and is the usual form of what is described as concentrated
sulfuric acid. Other concentrations are used for different purposes. Some
common concentrations are:
·
10%: Dilute sulfuric
acid for laboratory use
·
33.5%: Battery acid (used in automotive batteries)
·
37.52%: Battery acid (used in traction, lift truck,
forklift batteries). 1.285 specific gravity (spgr).
·
62.18%: Chamber or
fertilizer acid
·
77.67%: Tower or Glover
acid
·
98%: Concentrated
acid
Since sulfuric acid is a strong
acid, a 0.50 M solution of sulfuric acid has a pH close to zero.
Safety:
Industrial
hazards
Although sulfuric acid is non-flammable, contact with metals in the
event of a spillage can lead to the liberation of hydrogen gas. The
dispersal of acid aerosols and gaseous sulfur dioxide is an additional
hazard of fires involving sulfuric acid. Water should not be used as the
extinguishing agent because of the risk of further dispersal of aerosols:
carbon dioxide is preferred where possible.
Sulfuric acid is not considered toxic besides its obvious corrosive
hazard, and the main occupational risks are skin contact leading to burns
(see below) and the inhalation of aerosols. Exposure to aerosols at high
concentrations leads to immediate and severe irritation of the eyes,
respiratory tract and mucous membranes: this ceases rapidly after exposure,
although there is a risk of subsequent pulmonary edema if tissue damage has
been more severe. At lower concentrations, the most commonly reported
symptom of chronic exposure to sulfuric acid aerosols is erosion of the
teeth, found in virtually all studies: indications of possible chronic
damage to the respiratory tract are inconclusive as of 1997. In the United
States, the permissible exposure limit (PEL) for sulfuric acid is fixed at
1 mg/m3: limits in other countries are similar. Interestingly
there have been reports of sulfuric acid ingestion leading to vitamin B12
deficiency with subacute combined degeneration. The spinal cord is most
often affected in such cases, but the optic nerves may show demyelination,
loss of axons and gliosis.
Laboratory
hazards
The corrosive
properties of sulfuric acid are accentuated by its highly exothermic
reaction with water. Hence burns from sulfuric acid are potentially more
serious than those of comparable strong acids (e.g. hydrochloric acid), as
there is additional tissue damage due to dehydration and particularly due
to the heat liberated by the reaction with water, i.e. secondary thermal
damage. The danger is obviously greater with more concentrated preparations
of sulfuric acid, but it should be remembered that even the normal
laboratory "dilute" grade (approx. 1 M, 10%) will char paper by
dehydration if left in contact for a sufficient length of time. Solutions
equal to or stronger than 1.5 M should be labeled CORROSIVE, while solutions
0.5 M but less than 1.5 M should be labeled IRRITANT. The standard first
aid treatment for acid spills on the skin is, as for other corrosive
agents, irrigation with large quantities of water: Washing should be
continued for a sufficient length of time—at least ten to fifteen
minutes—in order to cool the tissue surrounding the acid burn and to
prevent secondary damage. Contaminated clothing must be removed immediately
and the underlying skin washed thoroughly.
Preparation of
the diluted acid can also be dangerous due to the heat released in the
dilution process. It is essential that the concentrated acid is added to
water and not the other way round, to take advantage of the relatively high
heat capacity of water. Addition of water to concentrated sulfuric acid leads
at best to the dispersal of a sulfuric acid aerosol, at worst to an
explosion. Preparation of solutions greater than 6 M (35%) in concentration
is the most dangerous, as the heat produced can be sufficient to boil the
diluted acid: efficient mechanical stirring and external cooling (e.g. an
ice bath) are essential.
Potential Health Hazards
SKIN: Causes severe burns.
EYES: Liquid contact can cause
irritation, corneal burns, and conjunctivitis. May result in severe or
permanent injury. May cause blindness.
INHALATION: Inhalation of fumes or
acid mist can cause irritation or corrosive burns to the upper respiratory
system, including the nose, mouth and throat. May irritate the lungs. May
cause pulmonary edema.
INGESTION: Causes burns of the mouth,
throat and stomach. May be fatal if swallowed. Hazards are also applicable
to dilute solutions.
Polarity and conductivity
Anhydrous H2SO4
is a very polar liquid, with a dielectric constant of around 100. This is
due to the fact that it can dissociate by protonating itself, a process
known as autoprotolysis, which occurs to a high
degree, more than 10 billion times the level seen in water:
2 H2SO4 → H3SO4+
+ HSO4−
This allows protons to be highly
mobile in H2SO4. It also makes sulfuric acid excellent
for many reactions. In fact, the equilibrium is more complex than shown
above. 100% H2SO4 contains the following species at
equilibrium (figures shown as mol per kg solvent):
HSO4− (15.0), H3SO4+
(11.3), H3O+ (8.0), HS2O7−
(4.4), H2S2O7 (3.6), H2O (0.1)
Uses
A mixture of sulfuric acid and
water is used as the electrolyte in lead-acid battery where it undergoes a
reversible reaction where lead and lead dioxide are converted to lead(II)
sulfate.
Besides it’s use in batteries, sulfuric
acid is a very important commodity chemical. A nation's sulfuric acid
production is a good indicator of its industrial strength.
The major use (60% of total worldwide) for sulfuric acid is in the
"wet method" for the production of phosphoric acid, used for
manufacture of phosphate fertilizers as well as trisodium phosphate for
detergents. In this method phosphate rock is used, and more than 100
million tonnes is processed annually. This raw material is shown below as
fluorapatite, though the exact composition may vary. This is treated with
93% sulfuric acid to produce calcium sulfate, hydrogen fluoride (HF) and
phosphoric acid. The HF is removed as hydrofluoric acid. The overall
process can be represented as:
Ca5F(PO4)3 + 5 H2SO4
+ 10 H2O → 5 CaSO4•2 H2O + HF + 3
H3PO4
Sulfuric
acid is used in large quantities in iron and steel making principally as
pickling-acid used to remove oxidation, rust and scale from rolled sheet
and billets prior to sale into the automobile and white-goods business. The
used acid is often re-cycled using a Spent Acid Regeneration (SAR) plant.
These plants combust the spent acid with natural gas, refinery gas, fuel
oil or other suitable fuel source. This combustion process produces gaseous
sulfur dioxide (SO2) and sulfur trioxide (SO3) which
are then used to manufacture "new" sulfuric acid. These types of
plants are common additions to metal smelting plants, oil refineries, and
other places where sulfuric acid is consumed on a large scale, as operating
a SAR plant is much cheaper than purchasing the commodity on the open
market.
Ammonium
sulfate, an important nitrogen fertilizer is most commonly produced as a
by-product from coking plants supplying the iron and steel making plants,
Reacting the ammonia produced in the thermal decomposition of coal with
waste sulfuric acid allows the ammonia to be crystallised out as a salt
(often brown because of iron contamination) and sold into the
agro-chemicals industry.
Another important use for sulfuric
acid is for the manufacture of aluminium sulfate, also known as
papermaker's alum. This can react with small amounts of soap on paper pulp
fibres to give gelatinous aluminium carboxylates, which help to coagulate
the pulp fibres into a hard paper surface. It is also used for making
aluminium hydroxide, which is used at water treatment plants to filter out
impurities, as well as to improve the taste of the water. Aluminium sulfate
is made by reacting bauxite with sulfuric acid:
Al2O3 + 3 H2SO4
→ Al2(SO4)3
+ 3 H2O
Sulfuric
acid is used for a variety of other purposes in the chemical industry. For
example, it is the usual acid catalyst for the conversion of
cyclohexanoneoxime to caprolactam, used for making nylon. It is used for
making hydrochloric acid from salt via the Mannheim process. Much H2SO4
is used in petroleum refining, for example as a catalyst for the reaction
of isobutane with isobutylene to give isooctane, a compound that raises the
octane rating of gasoline (petrol). Sulfuric acid is also important in the
manufacture of dyestuffs.
Sulfuric
acid is also the principal ingredient in some drain cleaners, used to clear
blockages consisting of paper, rags, and other materials not easily
dissolved by caustic solutions.
Sulfuric
acid is also used as a general dehydrating agent in its concentrated form.
See Reaction with water.
Chemical properties
Reaction with water
The hydration reaction of sulfuric
acid is highly exothermic. If water is added to concentrated sulfuric acid,
it can boil and spit dangerously. One should always add the acid to the
water rather than the water to the acid. This can be remembered through
mnemonics such as "Always do things as you oughta, add the acid to the
water. If you think your life's too placid, add the water to the
acid", "A.A.: Add Acid", or "Drop acid, not
water." Note that part of this problem is due to the relative
densities of the two liquids. Water is less dense than sulfuric acid and
will tend to float above the acid. The reaction is best thought of as
forming hydronium ions, by:
H2SO4 + H2O → H3O+ +
HSO4-
And then:
HSO4- + H2O → H3O+ + SO42-
Other reactions
of sulfuric acid
As an acid, sulfuric acid reacts
with most bases to give the corresponding sulfate. For example, copper(II)
sulfate, the familiar blue salt of copper used for electroplating and as a
fungicide, is prepared by the reaction of copper(II) oxide with sulfuric
acid:
CuO + H2SO4 → CuSO4 + H2O
Sulfuric acid can be used to displace
weaker acids from their salts, for example sodium acetate gives acetic
acid:
H2SO4 + CH3COONa → NaHSO4 + CH3COOH
Likewise
the reaction of sulfuric acid with potassium nitrate can be used to produce
nitric acid, along with a precipitate of potassium bisulfate. With nitric
acid itself, sulfuric acid acts as both an acid and a dehydrating agent,
forming the nitronium ion NO2+, which is important in
nitration reactions involving electrophilic aromatic substitution. This
type of reaction where protonation occurs on an oxygen atom, is important
in many reactions in organic chemistry, such as Fischer esterification and
dehydration of alcohols.
Sulfuric acid reacts with most
metals in a single displacement reaction to produce hydrogen gas and the metal
sulfate. Dilute H2SO4 attacks iron, aluminium, zinc,
manganese and nickel, but tin and copper require hot concentrated acid.
Lead and tungsten are, however, resistant to sulfuric acid. The reaction
with iron (shown) is typical for most of these metals, but the reaction
with tin is unusual in that it produces sulfur dioxide rather than
hydrogen.
Fe(s) + H2SO4(aq) → H2(g) + FeSO4(aq)
Sn(s) + 2 H2SO4(aq) → SnSO4(aq) + 2 H2O(l)
+ SO2(g)
Manufacture
Sulfuric acid is produced from
sulfur, oxygen and water via the contact process.
In the first step, sulfur is
burned to produce sulfur dioxide.
(1) S(s) + O2(g) → SO2(g)
This is then oxidised to sulfur
trioxide using oxygen in the presence of a vanadium(V) oxide catalyst.
(2) 2 SO2 + O2(g) → 2 SO3(g)
(in presence of V2O5)
Finally, the sulfur trioxide is
treated with water (usually as 97-98% H2SO4
containing 2-3% water) to produce 98-99% sulfuric acid.
(3) SO3(g) + H2O(l) → H2SO4(l)
Note that directly dissolving SO3
in water is impractical due to the highly exothermic nature of the
reaction. Mists are formed instead of a liquid. Alternatively, the SO3
is absorbed into H2SO4 to produce oleum (H2S2O7),
which is then diluted to form sulfuric acid.
(3) H2SO4(l) + SO3 → H2S2O7(l)
S-Phrases / Sulfuric Acid
S1: Keep locked up.
S2: Keep out of the reach of children.
S26: In case of contact with eyes, rinse immediately
with plenty of water and seek medical advice.
S30: Never add water to this product.
S45: In case of accident or if you feel unwell, seek
medical advice immediately (show the label where possible).
 Back to top Back to top
Have questions? Contact TECH SUPPORT:
Corporate
|
About Us
| Site Map | Services | Clients | Global Presence
| Email: Parts &
Service | Battery Care
Privacy | Terms of Use | Ethics | Legal
 
Powered by: Sun Microsystems
© Copyright GB Industrial Battery - All rights reserved.
Last Updated: Monday, December 03,
2007 - 7:18 AM Eastern Time.
 
|
|