|
One of the key
parameters of battery operation is the specific gravity of the electrolyte.
Specific gravity is the ratio of the weight of a solution (sulfuric
acid in this case) to the weight of an equal
volume of water at a specified temperature. This measurement is usually measured using a Hydrometer. The specific gravity of a fully charged GB
Industrial Battery is the industry standard of 1.285.
Specific gravity is
used as an indicator of the state of charge of a cell or battery. However,
specific gravity measurements cannot determine a battery's capacity.
During discharge, the specific gravity
decreases linearly with the ampere-hours discharged as indicated in the
illustration below.
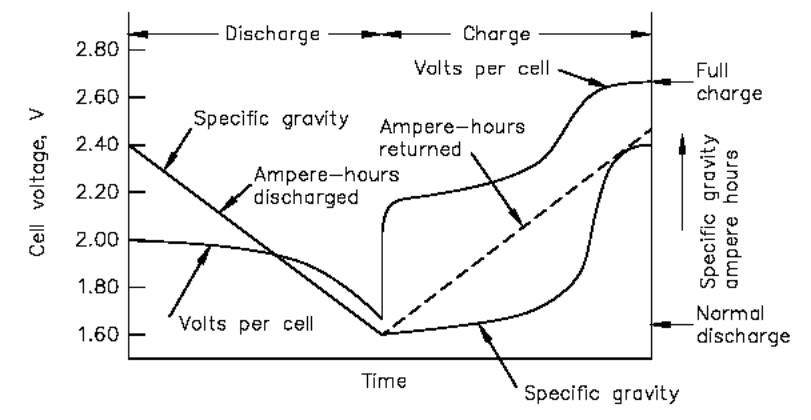
Changes in voltage and specific gravity during charge
and discharge.
Therefore, during fully charged steady-state
operation and on discharge, measurement of the specific gravity of the electrolyte
provides an approximate indication of the state of charge of the cell. The
downward sloping line for the specific gravity during discharge is
approximated by the equation below:
Specific gravity = single-cell
open-circuit voltage - 0.845 (example:
2.13v – 0.845 = 1.285)
Or
Single-cell open circuit voltage =
specific gravity + 0.845.
The above equations permit electrical
monitoring of approximate specific gravity on an occasional basis. As
mentioned earlier, specific gravity measurements cannot be taken on sealed
lead-acid batteries. Measurement of the cell open-circuit voltage has been
used as an indicator of the state of charge of a sealed battery. More
reliable methods for determining the state of charge of sealed batteries
are under development.
The specific gravity decreases during the
discharging of a battery to a value near that of pure water and it
increases during a recharge. The battery is considered fully charged when
specific gravity reaches its highest possible value.
Specific gravity varies with temperature
and the quantity of electrolyte in a cell. When
the electrolyte is near the low-level mark, the specific gravity is higher
than nominal and drops as water is added to the cell to bring the
electrolyte to the full level. The volume of electrolyte expands as temperature
rises and contracts as temperature
drops, therefore affecting the density or
specific gravity reading. As the volume of electrolyte expands, the
readings are lowered and, conversely, specific gravity increases with
colder temperatures.
The specific gravity for a given battery is
determined by the application it will be used in, taking into account
operating temperature
and battery life. Typical specific gravities for
certain applications are shown in Table 1.
|
Specific Gravity
|
Applications
|
|
1.285
|
Heavily cycled
batteries such as for forklifts (traction).
|
|
1.260
|
Automotive (SLI)
|
|
1.250
|
UPS – Standby with high momentary
discharge current requirement.
|
|
1.215
|
General applications such as power
utility and telephone.
|
Table 1
As mentioned earlier,
the specific gravity (spgr.) of a fully charged industrial battery,
or traction battery, is generally 1.285, depending on the manufacturer
and type. Some manufacturers use specific gravities as high as 1.320 in an
attempt to gain additional Ah capacity, but at the cost of a shorter cycle
life.
Represented in Table
2 (below), the electrolyte in a fully charged battery is still 62.48%
water. Higher gravity acid, such 1.600 spgr, can be used to
adjust the gravity of batteries that have been diluted due to repeated
overflow caused over-filling. Note: Acid adjustments should only be
performed by factory-trained technicians in a controlled environment.
|
% Sulfuric Acid
|
% Water
|
Specific Gravity (68°F)
|
|
37.52
|
62.48
|
1.285
|
|
48
|
52
|
1.380
|
|
50
|
50
|
1.400
|
|
60
|
40
|
1.500
|
|
68.74
|
31.26
|
1.600
|
|
70
|
30
|
1.616
|
|
77.67
|
22.33
|
1.705
|
|
93
|
7
|
1.835
|
Table 2
In the selection of a battery for a given
application, some of the effects of high or low specific gravity to be
considered:
|
Higher Gravity =
|
Lower Gravity =
|
|
More capacity
|
Less capacity
|
|
Shorter life
|
Longer life
|
|
Higher momentary discharge rates
|
Lower momentary discharge rates
|
|
Less adaptable to "floating:
operation
|
More adaptable to "floating"
operation
|
|
More standing loss
|
Less standing loss
|
Table 3
A solution of
higher specific gravity is heavier per unit volume than one of lower
specific gravity. Therefore the more concentrated electrolyte created
during charging sinks to the bottom of the battery jar creating a gradient
in specific gravity. The gassing that occurs on overcharge serves as a
"mixer" and makes the specific gravity uniform throughout the
cell. To avoid erroneous readings, specific gravity measurements should
only be taken after an equalizing charge and subsequent float charge for at
least 72 hours.
 Back to top Back to top
|