EDTA
|
EDTA |
|
|
Chemical name |
EDTA |
|
Other names |
EDTA |
|
Chemical formula |
C10H16N2O8 |
|
Molecular mass |
292.25 g/mol |
|
CAS number |
[60-00-4] |
|
Density |
0.86 g/cm³ |
|
Melting point |
237-245 °C (dec.) |
|
SMILES |
OC(CN(CC(O)=O)C- CN(CC(O)=O)CC(O)=O)=O |
|
Hazards |
|
|
Main hazards |
irritant |
|
NFPA 704 |
1 0
|
|
R/S statement |
R: 36 |
|
RTECS number |
AH4025000 |
|
Disclaimer and references |
|
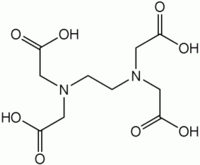
EDTA is a popular acronym for the chemical compound ethylenediaminetetraacetic
acid. EDTA refers to the chelating agent that is widely used to sequester
di- and trivalent metal ions. EDTA features four carboxylic acid and two amine
groups that can all bind to metals. EDTA forms specially strong complexes with
Mn(II), Cu(II), Fe(III), and Co(III).
Popular vs. chemical nomenclatureTo describe EDTA and its various protonated forms,
chemists use a more cumbersome but more precise acronym that distinguishes
between EDTA4−, the conjugate base that is the ligand, and H4EDTA,
the precursor to that ligand.
Coordination chemistry principles
In coordination chemistry, H4EDTA is a
member of the aminocarboxylate family of ligands that includes imidodiacetic
acid ("H2IDA") and nitrilotriacetic acid ("H3NTA").
More specialized relatives include N,N'-ethylenediaminediacetic acid ("H2EDDA")
and 1,2-diaminocyclohexane-N,N,N',N'-tetraacetic acid ("H4CyDTA").
These ligands are all formally derived from the amino acid glycine.
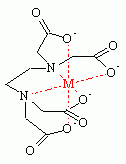
H4EDTA forms highly stable coordination
compounds that are soluble in water. In these complexes, the ligand is usually
either hexa- or pentadentate, EDTA4− or HEDTA3−,
respectively. Such complexes are chiral, and [Co(EDTA)]− has
been resolved into enantiomers.
Metal-EDTA chelate
Uses
Annual consumption of EDTA is about 35,000 tons in
1999 in Europe and 50,000 tons in the US. The most important uses are:
- Industrial cleaning:
complexation of Ca2+ and Mg2+ ions, binding of heavy
metals.
- Detergents: complexation of
Ca2+ and Mg2+ (reduction of water hardness).
- Photography: use of
Fe(III)EDTA as oxidizing agent.
- Pulp and paper industry:
complexation of heavy metals during chlorine-free bleaching, stabilization
of hydrogen peroxide.
- Textile industry:
complexation of heavy metals, bleach stabilizer.
- Agrochemicals: Fe, Zn and
Cu fertilizer, especially in calcareous soils.
- Hydroponics: iron-EDTA is used
to solubilize iron from in nutrient solutions.
More specialised uses of EDTA are:
- Food: added as preservative
to prevent catalytic oxidation by metal ions or stabilizer and for iron
fortification.
- Personal care: added to cosmetics
to improve product stability.
- Oil production: added into
the borehole to inhibit mineral precipitation.
- Dairy and beverage
industry: cleaning of bottles from milk stains.
- Flue gas cleaning: removal
of NOx.
- Medicine: used in chelation
therapy (brand name Endrate®, marketed by Hospira; generic product is also
on the market) for acute hypercalcemia and mercury poisoning and has been
used for lead poisoning.
- Dentistry as a root canal
irrigant to remove organic and inorganic debris (smearlayer).
- Soft drinks containing ascorbic
acid and sodium benzoate, to mitigate formation of benzene (a carcinogen).
- Recycling: recovery of used
lead acid batteries.
In laboratory science, EDTA is also used for:
- Scavenging metal ions: in biochemistry
and molecular biology, ion depletion is commonly used to inactivate
metal-dependent enzymes which could damage DNA or proteins
- Complexometric titrations.
- Buffer solutions.
- Determination of water
hardness.
- Used in medical and
laboratory equipment as an anticoagulant.
To describe EDTA and its various protonated forms,
chemists use a more cumbersome but more precise acronym that distinguishes
between EDTA4−, the conjugate base that is the ligand, and H4EDTA,
the precursor to that ligand.
Coordination chemistry principles
In coordination chemistry, H4EDTA is a
member of the aminocarboxylate family of ligands that includes imidodiacetic
acid ("H2IDA") and nitrilotriacetic acid ("H3NTA").
More specialized relatives include N,N'-ethylenediaminediacetic acid ("H2EDDA")
and 1,2-diaminocyclohexane-N,N,N',N'-tetraacetic acid ("H4CyDTA").
These ligands are all formally derived from the amino acid glycine.
H4EDTA forms highly stable coordination
compounds that are soluble in water. In these complexes, the ligand is usually
either hexa- or pentadentate, EDTA4− or HEDTA3−,
respectively. Such complexes are chiral, and [Co(EDTA)]− has
been resolved into enantiomers.
Metal-EDTA chelate
Uses
Annual consumption of EDTA is about 35,000 tons in
1999 in Europe and 50,000 tons in the US. The most important uses are:
- Industrial cleaning:
complexation of Ca2+ and Mg2+ ions, binding of heavy
metals.
- Detergents: complexation of
Ca2+ and Mg2+ (reduction of water hardness).
- Photography: use of
Fe(III)EDTA as oxidizing agent.
- Pulp and paper industry:
complexation of heavy metals during chlorine-free bleaching, stabilization
of hydrogen peroxide.
- Textile industry:
complexation of heavy metals, bleach stabilizer.
- Agrochemicals: Fe, Zn and
Cu fertilizer, especially in calcareous soils.
- Hydroponics: iron-EDTA is used
to solubilize iron from in nutrient solutions.
More specialised uses of EDTA are:
- Food: added as preservative
to prevent catalytic oxidation by metal ions or stabilizer and for iron
fortification.
- Personal care: added to cosmetics
to improve product stability.
- Oil production: added into
the borehole to inhibit mineral precipitation.
- Dairy and beverage
industry: cleaning of bottles from milk stains.
- Flue gas cleaning: removal
of NOx.
- Medicine: used in chelation
therapy (brand name Endrate®, marketed by Hospira; generic product is also
on the market) for acute hypercalcemia and mercury poisoning and has been
used for lead poisoning.
- Dentistry as a root canal
irrigant to remove organic and inorganic debris (smearlayer).
- Soft drinks containing ascorbic
acid and sodium benzoate, to mitigate formation of benzene (a carcinogen).
- Recycling: recovery of used
lead acid batteries.
In laboratory science, EDTA is also used for:
- Scavenging metal ions: in biochemistry
and molecular biology, ion depletion is commonly used to inactivate
metal-dependent enzymes which could damage DNA or proteins
- Complexometric titrations.
- Buffer solutions.
- Determination of water
hardness.
- Used in medical and
laboratory equipment as an anticoagulant.