|

|

|
Plates
The principle of the lead acid cell can be demonstrated with simple
sheet lead plates for the two electrodes. However such a construction would
only produce around an amp for roughly postcard sized plates, and it would
not produce such a current for more than a few minutes.
A plate consists of a rectangular lead plate alloyed with a little
antimony to improve the mechanical characteristics. The plate is in fact a
grid with rectangular holes in it, the lead forming thin walls to the
holes. The holes are filled with a mixture of red lead and 33% dilute
sulphuric acid (Different manufacturers have modified the mixture). The
paste is pressed into the holes in the plates, which are slightly tapered
on both sides to assist in retention of the paste. This paste remains
porous and allows the acid to react with the lead inside the plate
increasing the surface area many fold. At this stage the positive and negative
plates are identical. Once dry the plates are then stacked together with
suitable separators and inserted in the battery container. An odd number of
plates are always used, with one more negative plate than positive. Each
alternate plate is connected together. After the acid has been added to the
cell, the cell is given its first forming charge. The positive plates
gradually turn the chocolate brown color of Lead Dioxide, and the negative
turn the slate gray of 'spongy' lead. Such a cell is ready to be used.
One of the problems with the plates in a lead-acid battery is that
the plates change size as the battery charges and discharges, the plates
increasing in size as the active material absorbs sulphate from the acid
during discharge, and decreasing as they give up the sulphate during
charging. This causes the plates to gradually shed the paste during their
life. It is important that there is plenty of room underneath the plates to
catch this shed material. If this material reaches the plates a shorted
cell will occur.
The grid structure in both pasted and tubular plate batteries is
made from a lead alloy. A pure lead grid structure is not strong enough by
itself to stand vertically while supporting the active material. Other
metals in small quantities are alloyed with lead for added strength and
improved electrical properties. The most commonly alloyed metals are
antimony, calcium, tin, and selenium.
The two most common alloys used today to harden the grid are antimony
and calcium. Batteries with these types of grids are sometimes called
“lead-antimony” and & “lead-calcium” batteries. Tin is added to
lead-calcium grids to improve cyclability. The major differences between
batteries with lead-antimony and lead-calcium grids are as follows:
Lead-antimony batteries can be deep cycled more times than
lead-calcium batteries.
Flooded lead-antimony batteries require more frequent maintenance
as they near end-of-life since they use an increasing amount of water and
require periodic equalization charges.
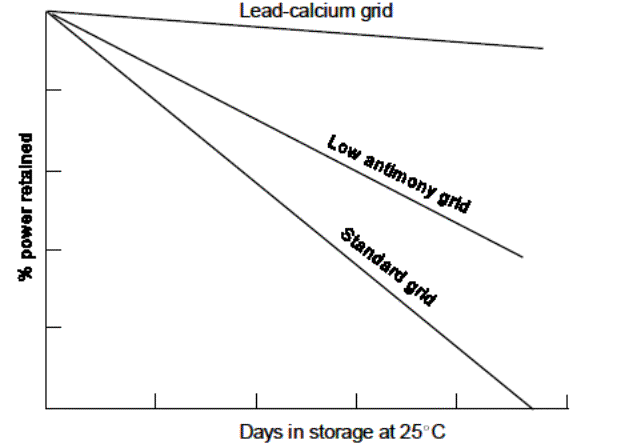
Lead-calcium batteries have lower self-discharge rates as shown in
the illustration below and therefore, will draw less current while on float
charge than lead-antimony batteries.
Lead-calcium positive plates may grow in length and width because
of grid oxidation at the grain boundaries. This oxidation is usually caused
by long-term overcharging, which is common to UPS and other batteries on
constant-float changing. Grids may grow in size sufficiently to cause
buckling or rupture of their containers.
Another type of grid alloy is lead-selenium. In reality, this
battery is actually a low lead-antimony grid with a slight amount of
selenium. Lead-selenium has characteristics that fall somewhere between
lead-calcium and lead-antimony.
When pure lead is mixed with an alloy there may be undesirable
characteristics introduced in the performance of the battery.
 Back to top Back to top
|
Have questions? Contact TECH SUPPORT:
Corporate
|
About Us | Site Map | Services | Clients | Global Presence
| Email: Parts &
Service | Battery Care
Privacy |
Terms of Use |
Ethics |
Legal
© Copyright GB Industrial Battery - All rights reserved.
Last Updated: Monday, December 03, 2007 - 5:53 AM Eastern Time.
 
|